3 - Periodic Table and Electron Shells
Abstract (TL;DR):
This lesson introduces the periodic table and the concept of electron shells, both key components to how atoms interact with each other. The concept of electron shells, originally theorized by Niels Bohr, are divided into innter shells and outer shells, called valence shells.
Image via MicroMountain
And we’re back with some more. This time, finally, we discuss orbitals, teased in the first section.
What if I told you that you already know what they are? For those of you who have already studied chemistry, you’ve already might have started to put together the pieces from the last two lessons. For the rest of you, we have laid the groundwork for orbitals already by discussing what they are in detail. Orbitals are nothing more than the position of electrons in the space of an atom.
But, before we go over orbitals, let’s summarize the past lessons. Of course, if you want to understand these at a higher level, please check out part 1 and part 2.
In the Name of Energy
Low energy means no problems. The first lesson on electrons focused on their orientation and how they exist in a way that decreases their energy. The second furthered that discussion, showing that microscopic particles exhibit both particle-like behavior and wave-like behavior, changing the number of possible places that electrons can exist within the three dimensions, again, to minimize energy. In both lessons, we have discussed that modeling exactly where the electron is is difficult, but telling where it might be in a “cloud” of possible positions is far easier.
I don’t exaggerate when I say that, if you understand those two things, you understand most of orbital theory. But, of course, I’ll show you what I mean.
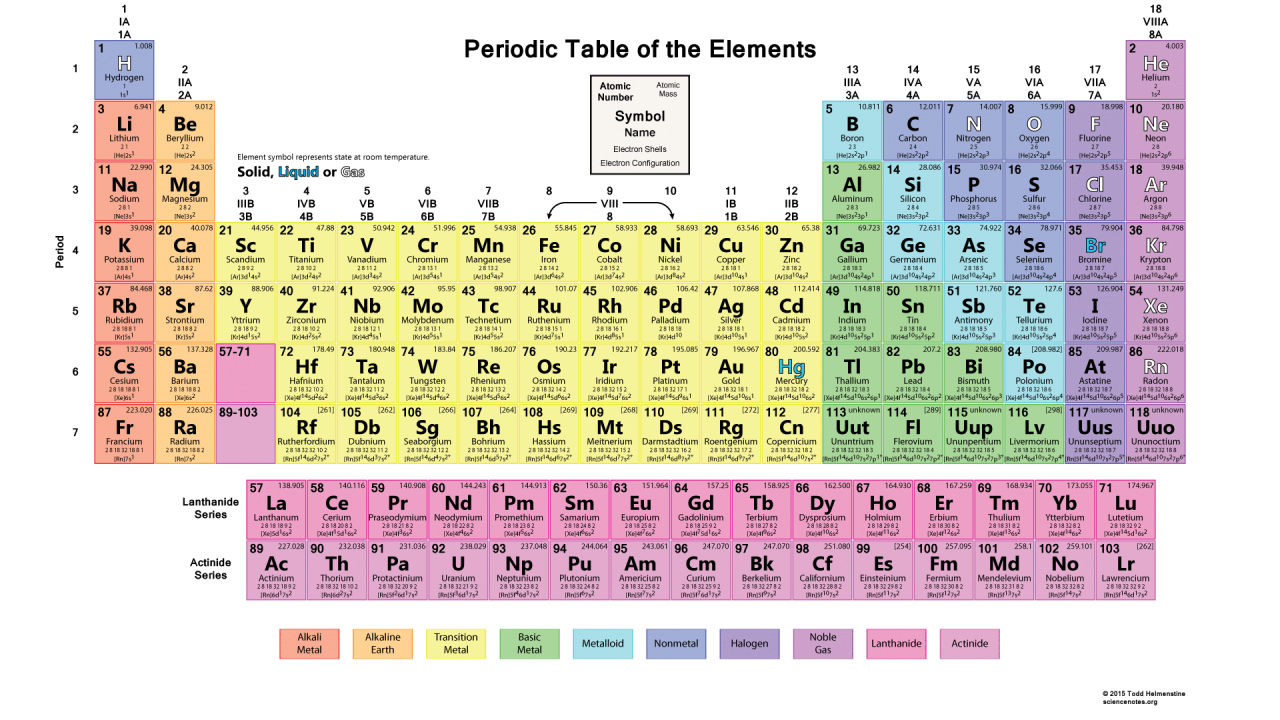
The Periodic Table of Elements
First, we should establish a familiarity with the periodic table.
Image via Science Notes
A nice, organized table, isn’t it?
This table, like most periodic tables should, gives us all of the information required to talk about the elements. The key, positioned in the top center, explains what is within each box, which we will go over in detail. First, the number in the top left, or the atomic number, represents the number of protons within the atom. Given no excess in positive or negative charge, the atomic number can also be taken to refer to the number of electrons within an atom. The number in the top right, or the atomic mass, is given as the mass of an atom. Since the nucleus of any atom makes up most of the weight, you may sometimes see a reference to the mass number given before any particle. The mass number, unlike the atomic number, is the combined amount of protons and neutrons in any element.
Image via IPod Physics
This is the nuclear notation, also known as AZE notation, where “A” is the mass number, Z is the atomic number, and E is the element symbol.
Elements will always have the same amount of protons, which is why the atomic number is defined as the number of protons. However, an element will not always have the same number of electrons or the same number of neutrons.
Image via TutorCircle
You might have heard the term “isotope” before. Isotopes are the variations of an elements, given by differing numbers of neutrons. Some isotopes are less stable than others and, the less stable they are, the less likely that they are occur less in nature.
As you can see, protium, the hydrogen with one proton only, is the most commonly occurring, and, as such, the mass number of hydrogen is usually written as 1. Isotopes of hydrogen can have a mass number of 2 or 3.
But now we get to the interesting and most relevant part of the table: the electron shells and configurations shown at the bottom.
Electron Shells
Remember when I said in the first lesson that reactions between elements exist in a give-and-take relationship? It’s finally time to tie the neat bow on the present that I had been preparing for you since the beginning. Let’s jump right in.
It is the “electron shell” that determines how many electrons an atom gives up. The idea of electron shells came from Niels Bohr, who believed that electrons needed to be a certain distance away from the nucleus.
The outermost electron shell is called the valence shell. This is the shell that interacts with the valence shells of other elements. We have determined through experimentation that the preferred number of valence electrons for any element in the valence shell is either two or eight, with very few exceptions. This is according to the amount of electrons within elements in the noble gas category (the elements on the rightmost column of the periodic table). The reason for that is due to a unique characteristic of noble gases – they are almost completely nonreactive. If you put a noble gas next to any element, without any further additions, nothing would happen.
While the outermost shell is important, however, I would like to turn your attention to the inner shells – where the orbitals truly shine…in the next lesson.
Keep that periodic table handy. And feel free to review and ask questions in preparation for the next lesson.